Our White Paper
A better future for all…
A “Best Choice Medicine” (BCM) Route to Drug Development to Solve the Aging-Associated Non-Communicable Disease Burden
The objective of this white paper is to propose a new modality called Best Choice Medicine (BCM). BCM provides potentially life-saving experimental treatments to patients suffering from Aging-Associated Non-Communicable Diseases (AA-NCDs) who would otherwise not survive without treatment or otherwise have a disease without curative options.
Simultaneously, BCM could drastically expedite the drug approval process by generating data from these patients.
Every day thousands of patients die from aging-associated non-communicable diseases (AA-NCDs) that might have been saved by new treatments that the United States Food and Drug Association (U.S. FDA) has no framework to allow them to try.
The process of aging is accompanied by changes at the cellular level, leading to an increased risk of death with time , [1] though it is debated if it is a regulated or adaptive process [2].
Recognized by the United Nations as a “threat to global development,” a mandate has been set to improve the consequences of such diseases [3]. As new drug candidates are developed to treat aging, more people with money will flee our regulatory systems in favor of treatments abroad. Inequity of access to medicine is dawning and likely only to broaden.
Understanding the medical tourism model is essential to understanding the wider impact that it offers and how it can be harnessed to meet people’s needs while benefiting future populations.
BCM would allow the same ease of access that Medical Tourism offers whilst allowing for cost-efficient safety and efficacy case studies within the United States. BCM will complement the U.S. FDA by giving thousands of patients access to cutting-edge medicine while concurrently expediting technological advancements to millions in need.
Approval and marketing of new drugs in the U.S. today costs about $2.6 billion and takes over 12 years before the drugs can be provided to patients [4]. Meanwhile, millions of people die globally that could be saved. To avoid being another statistic. Many patients and their doctors are being driven to offshore locations to pioneer promising new technologies in a new trend called “Medical Tourism.”
This paper reviews the benefits and drawbacks of the medical tourism trend versus creating a new regulatory route to bring new innovations back to the U.S. and maintain the country’s innovative edge. Understanding how the ease of access of the medical tourism model is an illustration of what can happen when biotech companies and governments cooperate.
An ethical and commercial imperative, the picture would encourage the establishment of a new regulatory route within the U.S. Previous studies on customer choices in medical tourism came down to the patient’s perception of the country they were traveling to, price, quality of facilities, quality of care, and physician training [5].
The present “do no harm” mindset of the FDA might be causing more harm than it is meant to prevent. Numerous patients have fought to try new medicines, as seen in the "Right to Try Act" legislation; alas, these laws do not cover newer and more precise genetic therapies which are not currently in clinical trials [6].
A new regulatory designation Regenerative Medicine Advanced Therapy (RMAT) addresses regenerative medicine but not early access to the treatments in the ever-demanding aging patient population (Section 3033 of the 21st Century Cures Act) [7].
Understanding the medical tourism model as an ethical business case for biotech companies could also help establish new regulatory routes within the United States.
BCM could give millions of patients access to technological advancements earlier than current legislation allows, letting biotech companies conduct first-in-human studies based on patient consent and a medical doctor’s approval. If such a new regulatory route was developed, these therapies could be given within the regulatory system and exponentially expedite therapeutic research and treatment in the United States.
The BCM plan proposed here could give millions of patients access to technological advancements earlier in drug development than current legislation allows and enable biotech companies to legally do first-in-human studies based on patient consent and a medical doctor’s approval to treat.
Globally, 14-16 million people traveled for medical services in 2017; the area is growing at a CAGR of approximately 8.8% annually. Spending in 2015 alone was estimated at $70 million and predicted to climb upward of $28 billion by 2024 [8]. Bringing medical innovations back to the U.S. first requires finding ways to reduce clinical research costs.
This obstacle is due to the excessive standards set by the FDA and other regulatory agencies throughout the world. Providing humans with the opportunity to try potential drugs and treatments on themselves prior to pre-clinical results not only offers these patients a chance to survive their terminal illnesses, but it would also tremendously reduce research costs [9] and provide invaluable data for the researchers.
Medical Tourism offers the opportunity to test new drugs and therapies directly in humans who would otherwise die without the drug or therapy. Plus, investment for further testing, production, and marketing would be easier to obtain if biotech companies had the initial human data before seeking funding for expensive clinical trials.
The risk in patients pursuing the medical tourism route is that not every treatment or provider has credibility. Patients can find themselves becoming guinea pigs for research that has nothing to do with treating their disease. Therefore, initially at least, BCM will be a clearinghouse for reviews of the various
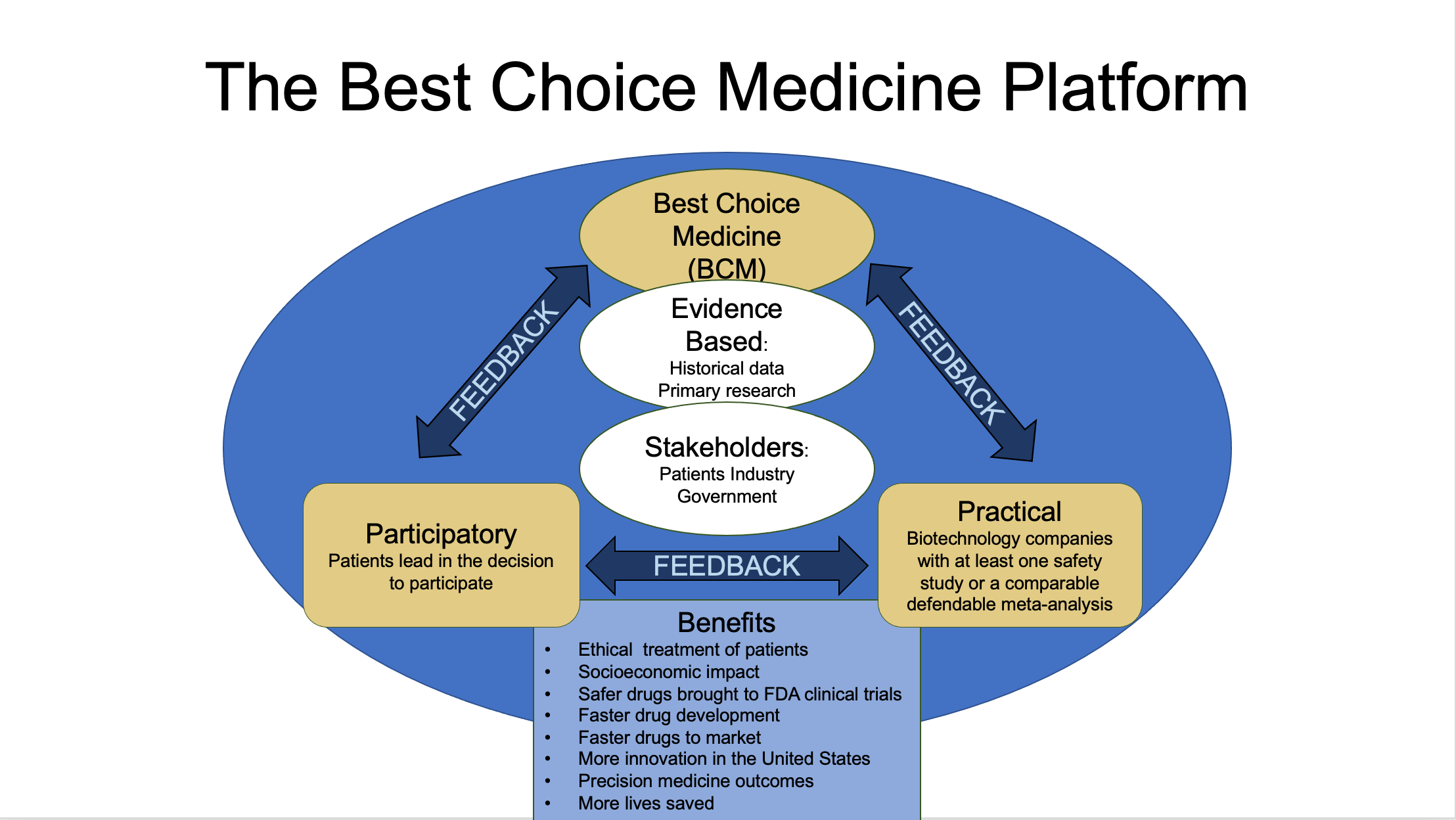
treatment options and providers that are available to the patients. Performing such a function requires no regulatory approval. At first BCM will be a virtual non-profit service, available to patients, composed of three review boards and four databases virtual nonprofit service composed of three review boards and four databases (Figure 1).
The ”Patient” database will be a database of the patients themselves and will contain relevant information compiled by the patients, their personal doctors, and/or their Powers of Attorney. The other three databases will be compiled by the Review Boards and will be available to be searched by the patients.
Likewise, the Patient database will be available to be searched by “Treatment Providers” with the approval of the patients. Treatment Providers will include treatment inventors (both academic and commercial), biotech/pharmaceutical companies, treatment suppliers, medical personnel, clinics, hospitals, etc.
The three Review Boards will review “Treatment Options”. The Boards will not only review Treatment Options submitted to BCM by Treatment Providers but will also review the scientific and medical field worldwide for additional Treatment Options. Treatment Options will include not only the treatments themselves but also the medical personnel, clinics, and hospitals.
The Review Boards will not make recommendations or provide approvals. On the other hand, Review Boards will recommend against Treatment Options if the boards find no credibility in the Treatment Option.
After reviewing Treatment Options, the Review Boards will simply summarize the pros and cons resulting from each review and save each summary to the relevant Databases.
Review Boards will also monitor the subsequent performance of Treatment Options and continually update their summaries with the information learned.
Review Board members will be available for patients to ask questions about the summaries.
Since BCM will not make recommendations BCM will be able to operate worldwide including in the U.S.
Review Board members will be assessed, approved, and reviewed by other members of the Review Boards. Assessment will be based on:
Skill at the critical meta-analysis of peer-reviewed as well as non-reviewed studies.
Logic, statistical theory, probability, and basic common sense.
Knowledge of biology, chemistry, and disease mechanisms.
Skill in experimental design and data analysis.
The ”Patient” database will be a database of the patients themselves and will contain relevant information compiled by the patients, their personal doctors, and/or their Powers of Attorney. The other three databases will be compiled by the Review Boards and will be available to be searched by the patients.
Likewise, the Patient database will be available to be searched by “Treatment Providers” with the approval of the patients. Treatment Providers will include treatment inventors (both academic and commercial), biotech/pharmaceutical companies, treatment suppliers, medical personnel, clinics, hospitals, etc.
The three Review Boards will review “Treatment Options.” The Boards will not only review Treatment Options submitted to BCM by Treatment Providers but will also review the scientific and medical field worldwide for additional Treatment Options. Treatment Options will include not only the treatments themselves but also the medical personnel, clinics, and hospitals.
The Review Boards will not make recommendations or provide approvals. On the other hand, Review Boards will recommend against Treatment Options if the boards find no credibility in the Treatment Option.
After reviewing Treatment Options, the Review Boards will simply summarize the pros and cons resulting from each review and save each summary to the relevant Databases.
AA-NCDs are responsible for 63% of all deaths worldwide [10, 11]. According to the 2019 Congressional Budget Office, AA-NCDs cost the US government around $1.5 trillion in 2018. Promising solutions for treating these diseases can be found in novel experimental regenerative medicine protocols, including gene therapies, stem cell and exosome treatments, small molecule drugs, regulated natural products, etc. But the high costs and stringent regulations to take advantage of these protocols necessitates traveling to other countries to benefit from the lower costs and relaxed regulations provided by medical tourism.
Concurrent with its aforementioned efforts, BCM plans to lobby for regulatory changes to eventually provide the same cost and regulatory benefits, offered by medical tourism, in the U.S. The United States Food and Drug Association (FDA) is a top-down power system established to help the government create food and drug standards.
Designed to protect, the FDA was founded on precautionary principles. Personal risk aversions within the agency hinder development as commissioners can destroy their careers if an approval goes awry. Restructuring the FDA has been considered, including changes allowing the agency to report directly to the President in the hope of avoiding political roadblocks [12].
BCM proposes to identify a radical paradigm shift that presents combined pragmatic and humanistic approaches to patients' needs, granting individuals the use of self-directing agency and opportunities to make decisions for themselves in a purposeful manner [12].
As an aging population expands, it is socially responsible, economically sound, and increasingly urgent to address the diseases of aging. But the US FDA does not consider aging a disease; this limits researchers from applying for grants to study aging directly. A trial cannot be conducted without a measurable endpoint, and though we all know what aging is, there is still no undisputed way to measure it.
In 2013, a peer-reviewed paper in Cell called “The Hallmarks of Aging” was released, citing nine different cellular processes that change in humans as the result of aging [13]. But a universally accepted way to measure aging is still not clear, although aging is undeniably real. Clinical studies must still rely on treating limitless numbers of specific diseases associated with aging rather than aging itself.
This only contributes to further delays in an already slow regulatory process.
And with the enormous costs of preclinical animal studies, most biotech companies implode before reaching clinical trials. Medical tourism is an alternative. BCM, in the U.S. would allow a faster and more thorough understanding of innovative drugs and better outcomes for the patients, even more so than medical tourism outside the U.S.
BCM would eventually be housed under the Department of Health and Human Services as an agency separate from the U.S. FDA (Figure 2). This disconnect from the U.S. FDA would allow both agencies to have their own order and rules but still communicate to transfer data when successful drug candidates are found. Successful drug candidates would then be passed on to the U.S. FDA for fast-track approval without the added costs associated with the Prescription Drug User Fee Act (PDUFA).
The U.S. BCM would consist of U.S. government representatives who manage a patient database, ensuring that each patient signs off on two documents. One document of their current medical diagnosis/condition signed by both themselves and their treating physician. The second document would express written “informed consent” as acceptance to participate in experimental medicine that indemnifies all parties of adverse outcomes.
Unlike the Right to Try Act, drugs through the BCM will not need to have passed a Phase I trial; they can be based on critical meta-analysis, reproducible basic research, more advanced development, and/or primary evidence based on proof of principle under similar or related conditions.
Figure 2: Organization Chart for Drug Testing and Approval in the U.S. including Best Choice Medicine (BCM).
Through BCM first-in-human data would be generated faster. This could drive more innovation, jobs, and public interest into the sector. Government, society, industry, researchers, and most importantly patients, the most prominent stakeholders, would all benefit.
BCM is strongly needed for those with no curative options and for those that are terminally ill. Terminally ill patients are defined as patients given a diagnosis with no available “regulated” curative treatment. These patients, or their caregivers who have power of attorney, would register on a website overseen by the United States government BCM task team while pharmaceutical companies, biotechnology companies, and device companies would also register their technologies and protocols. The task team would then network them together (Figure 3). All companies would be allowed to participate regardless of their previous experience in FDA trials.
Over 41 million people will die this year of aging diseases that have been significantly slowed or halted in animal models. No drugs are inherently safe or efficacious in all people through today's regulatory body, as shown by multiple drugs historically being removed from the market after approval. Despite the vast precautionary measures taken by the FDA, some drugs approved by the FDA have still been found later to not be safe for patients.
Between 1998 and 2010, at least ten dangerous drugs, approved by the FDA, were recalled for causing liver damage, heart attacks, strokes, and multiple deaths [14]. According to the CDC, the opioid crisis alone has killed over 840,000 people since 1999. Likewise, clinical studies have even been approved by the FDA that have put patients at tremendous risks that the FDA should have been aware of and wasn’t.
These include gene therapy studies, for example, in which proper precautions were not taken to protect the patients from cancer-causing DNA alterations and deadly immune responses [15]. BCM plans to minimize the years of counterproductive testing while reviewing the credibility of treatment options from data and concepts that already exist.
Several genetic treatments have shown significant benefits in aging diseases in animal models. However, advancing these treatments to humans is stalled because of complex regulatory procedures and the lack of funding to follow these procedures.
The total cost of drug development at $2.6 Billion is too high and is not serving an aging society’s needs. AA-NCDs are now the biggest killers on the planet. Through smarter regulations, millions of lives and enormous amounts of money could be saved (Figure 4).
Figure 4: Comparison of FDA and BCM.
BCM will bring about six areas of stakeholder opportunity:
1. Patients get access to the newest candidates in drug development.
2. A reduction in research costs via condensed regulation criterion.
3. Reduction in delivery costs of therapies.
4. Reduction in bench-to-patient time giving consenting patients the dignity to participate.
5. Modernization of transparent marketing achieved by open-access information.
6. Improved investor participation in clinical development due to knowing how drugs work in humans.
Many of the roadblocks to new and innovative therapies lie within social norms and cognitive biases. BCM offers flexibility to roadblocks by giving the patient the role of the primary decision maker.
References and Suggested Reading
1. Harman, D., The aging process: major risk factor for disease and death. Proc Natl Acad Sci U S A, 1991. 88(12): p. 5360-3.
2. Schmeer, C., et al., Dissecting Aging and Senescence—Current Concepts and Open Lessons. Cells, 2019. 8(11).
3. Fitzmaurice, C., et al., Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol, 2019. 5(12): p. 1749-1768.
4. Marino, M., Z. Jamal, and M.A. Siccardi, Pharmaceutics, in StatPearls. 2022, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL).
5. Kim, S., C. Arcodia, and I. Kim, Critical Success Factors of Medical Tourism: The Case of South Korea. Int J Environ Res Public Health, 2019. 16(24).
6. Agarwal, R. and L.B. Saltz, Understanding the Right to Try Act. Clin Cancer Res, 2020. 26(2): p. 340-343.
8. Prince, R.A., The Coming Boom In Health Tourism (https://www.forbes.com/sites/russalanprince/2016/05/02/the-coming-boom-in-health-tourism/?sh=6206c7fa548f). May 2, 2016,.
9. Sertkaya, A., et al., Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials, 2016. 13(2): p. 117-26.
10. Cheng, X., et al., Population ageing and mortality during 1990-2017: A global decomposition analysis. PLoS Med, 2020. 17(6): p. e1003138.
11. About Global NCDs (https://www.cdc.gov/globalhealth/healthprotection/ncd/global-ncd-overview.html#:~:text=Noncommunicable%20diseases%20(NCDs)%2C%20such,an%20emerging%20global%20health%20threat).
12. Adashi, E.Y., R.S. Rajan, and I.G. Cohen, When science and politics collide: Enhancing the FDA. Science, 2019. 364(6441): p. 628-631.
13. López-Otín C, B.M., Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell, 2013. 153(6): p. 1194-1217.
14. Saleh, N., 10 dangerous drugs recalled by the FDA (https://www.mdlinx.com/article/10-dangerous-drugs-recalled-by-the-fda/lfc-4008).
15. Check, E., Gene therapy put on hold as third child develops cancer. Nature, 2005. 433(7026): p. 561-561.